A brief on the potential allergenicity and safety assessment of genetically modified crops.
Public awareness and concern about food allergens are growing. Allergenic reactions to proteins expressed in GM crops have been one of the prominent concerns among biotechnology critics and concern of regulatory agencies.
Allergen is a protein that elicits a hypersensitive immune reaction, and this capacity of allergen is known as allergenicity. These allergens are very small and resistant to heat, and acid, and show stomach enzyme degradation, atopic reactions in the form of hives and other skin responses and gastric distress, but severe reactions can result in death from anaphylaxis. While the crops used as staple foods contain tens of thousands of different proteins, relatively few are allergenic. However, genetic engineering to modify the genetic makeup of crop plants might alter their allergenic potential. Allergenic proteins may be introduced into newly developed GM crops if the transgene is from a source known to be allergenic.A well-known example is an experimental GM soybean engineered to contain a Brazil nut (a known allergenic food) methionine-rich protein that was found to cause an allergic reaction in patients sensitive to this nut. As a result, GM soybean was withdrawn from further development and has never been marketed. As a measure to prevent the introduction of allergens
When a gene is inserted its shows unintended effects because it modifies the expression of endogenous genes leading to either over-expression or under-expression of specific proteins. If the host plant contains known allergenic proteins, adding a new gene into it could increase the expression of the allergens and the plant may become more allergenic. It is also possible that the insertion of the DNA sequence creates additional new proteins that are allergenic to susceptible individuals. The potential for any protein to be a food allergen is difficult to predict with any accuracy. A decision tree that has been developed by the World Health Organization involves asking questions about whether the protein exhibits characteristics that might increase its probability of being an allergen. These include sequence‐relationship to other known allergens and the stability of the protein to gastric digestion. Such a decision tree, however, is only a guide and not a good rigorous predictor of allergenic potential or potency. For instance, a decision tree for Novel food is given below.
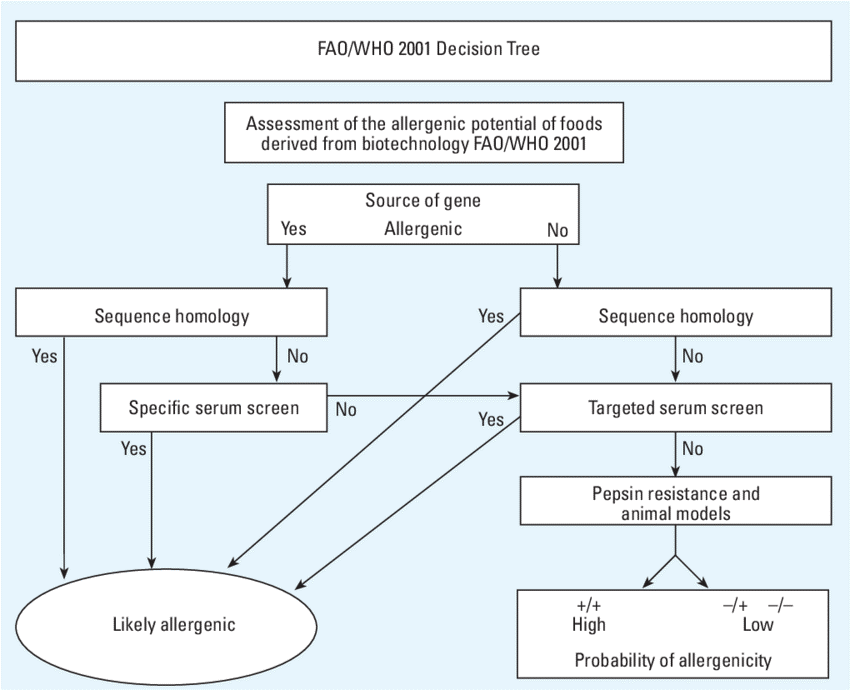
Safety Assessment of GM Food
Besides allergenicity, genetic modification might also alter the toxicity and chemical composition of a transgenic plant. Taking into account both intended and unintended changes that may occur due to genetic modification, the Codex Alimentarius Commission (Codex) has set out specific guidelines for the safety assessment of food derived from GM organisms. The FDA is not aware of any foods currently in the U.S. market from these types of genetically modified plant varieties, but the U.S. Food and Drug Administration issued a letter reminding developers and manufacturers of genetically modified plant varieties who intend to transfer genes for proteins that are food allergens (including allergens from foods identified as major food allergens– milk, eggs, fish, Crustacean shellfish, tree nuts, peanuts, wheat, Sesame and soybeans.) into new plant varieties used for food of the relevant legal requirements for these products. As per USFDA, if you are developing a genetically modified plant variety with a transferred gene that encodes a food allergen (which we also refer to as a “transferred allergen” for brevity), stewardship practices are likely to be more challenging and complicated than with other crops. When considering your proactive risk management plan, you will likely have to significantly bolster standard mitigation strategies and practices (e.g., segregation of crops) to provide the level of food safety assurance necessary to prevent inadvertent mixing of foods containing a transferred allergen with other foods.


To know more about allergens please click on the given link –
Developers would need to ensure that allergenicity information about an allotment of a particular crop containing a transferred allergen remains associated with the allotment through the supply chain (e.g., seed development, planting, harvesting, storage, transport, and processing) in a manner that enables responsible parties to ensure that the allotment is not mistaken for or used as its counterpart food that does not contain the allergen. Developers’ responsibility to market safe and properly labelled food and to meet the requirements under the Federal Food, Drug, and Cosmetic Act (FD&C Act).
National authorities of GM food-producing countries have adopted the comparative approach based on which to establish structured pre-market safety assessment schemes to evaluate the safety of food derived from genetic engineering. Any newly developed GM food should have undergone such assessment before approval for market sale. In cases where the potential hazard is identified from the assessment, the crop under evaluation should never be released for human consumption. GM food available in the international market has undergone safety assessment evaluation and is safe for human consumption.

Ms. Sonam Kumari, the author of this post is a passionate Biotechnologist and a Food Technologist, delving into the food sector to get fruitful results for benefit of the consumer and society. She is an Associate Consultant at Food Safety Works (Regulatory and Compliance team.)






